Seizing the Opportunity in the CGRP Targeting Drugs Market: The Prospects of Zavegepant
Three novel small molecules in the field of neuroscience were approved in 2023: a GABA-A positive allosteric modulator for postpartum depression developed by Sage Therapeutics, Zuranolone (Zurzuvae); a selective 5HT1A agonist for MDD marketed by Fabre-Kramer; and a CGRP receptor antagonist for migraine marketed by Pfize, Zavegepant (Zavpret).
The global pharmaceutical landscape is witnessing a remarkable surge in the development and approval of drugs targeting trigeminal neuropeptide calcitonin gene-related peptide (CGRP) or its receptor, especially for treating migraine, a debilitating neurological disorder affecting a substantial adult population worldwide. Among the various therapies emerging in this space, small molecule CGRP receptor antagonists, commonly known as “gepants”, have demonstrated a promising trajectory. This article seeks to provide an overview of the CGRP targeting drugs market, with a particular emphasis on the potential of the new gepant, Zavegepant, which, despite not having reported revenues yet, holds significant market promise.
The Migraine Market and CGRP Therapies
Migraine continues to be an underserved medical need, afflicting up to 15% of the adult population in most countries, more often affecting females. Characterized by incapacitating headache episodes and associated symptoms like photophobia, phonophobia, and autonomic disruptions, migraine calls for a demand for highly effective and targeted treatments.
CGRP-related therapies stand at the forefront, representing a paradigm shift from generic pain management to specified therapeutic approaches acting on the trigeminovascular system, with a specificity that translates to minimal adverse effects. Although chemically unrelated, small molecule CGRP receptor antagonists – gepants-have shown increased efficacy for the acute relief of migraine, contrasting the monoclonal antibodies designed for prophylaxis.
Monoclonal antibodies
In 2018, three monoclonal antibodies were approved:
● Erenumab (AMG-334 targeting CGRP receptor, an injection approved in 2018 for the prevention of migraine);
● Fremanezumab (an injection approved in 2018 for the prevention of migraine);
● and Galcanezumab (an injection approved in 2018 for the prevention of migraines and for the treatment of cluster headaches).
In 2020, another preventitive treatment eptinezumab was approved. It is an infusion every 3 months developed by Lundbeck Seattle Biopharmaceuticals.
Small molecules approved
● Ubrogepant (the first oral tablet approved in 2019 developed by Allergan);
● Rimegepant (an orally disintegrating tablet approved in 2020 developed by Pfizer);
● Atogepant (an oral tablet approved in 2021 developed by AbbVie)
● and Zavegepant (the first and only intranasal spray approved in 2023 developed by Pfizer)
Zavegepant: Another Novel Player by Pfizer
In March 2023, the landscape of migraine treatment was reshaped with the approval of Zavzpret (zavegepant), Pfizer's intranasal spray for acute migraine with or without aura in adults. It is currently the first and only intranasal spray CGRP antagonist. With ongoing investigations for its oral form in chronic migraine prevention, Zavegepant expands the gepants family, including successful predecessors like Ubrogepant (the first oral CRGP antagonist by Allergan/AbbVie) and Rimegepant (an orally disintegrating tablet).

Pfizer's acquisition of Biohaven in late 2022 not only furnished them with the wildly successful Nurtec ODT/Vydura (rimegepant) but also bestowed comprehensive CGRP programs, promising to steer the company to new zeniths within the arena of migraine prophylaxis and treatment.
Projecting Zavegepant's Market Potential
Despite Zavegepant's novelty and the absence of revenue reports, a projection of its market potential can be drawn by examining the performance of its gepant counterparts. This class of antagonists has demonstrated a robust market presence, with an observable year-on-year revenue increase, as noted in drugs like Ubrogepant, Rimegepant, and Atogepant. For instance, Rimegepant, also from Pfizer, generated 928 million USD in 2023.
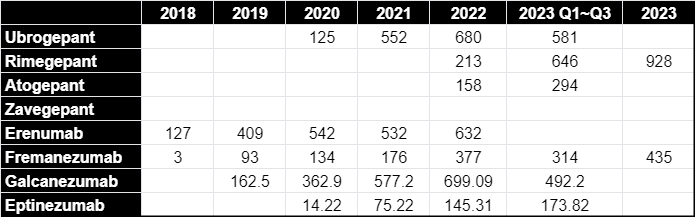
One can infer that Zavegepant, with its unique intranasal administration and backed by Pfizer's robust marketing machinery, is well-positioned to capture a significant share of this burgeoning market. By extrapolating the avialable revenue data of other CGRP targeting drugs, it is conceivable that Zavegepant could follow a simiar, if not more, lucrative trajectory, positioning itself as a cornerstone therapy in migraine management.
A Call to Action for Generic Drug Companies
Generic drug companies must seize the burgeoning opportunities in the CGRP antagonists market, with Zavegepant leading the way. As Unibest aspires to be the best partner for global pharmaceutical companies, we invite you to collaborate with us in the provision of development solutions and manufacturing for pharmaceutical products, gepant intermediates.

With our continuous investment in advanced technology, green chemistry, and R&D, coupled with our one-stop solution for the pharmaceutical industry, Unibest is ideally positioned to support your venture into the promising CGRP-targeting drugs market.
REFERENCES
Al-Hassany, L. et al. Future targets for migraine treatment beyond CGRP. J Headache Pain 24, 76 (2023).
Edvinsson, L. Role of CGRP in Migraine. in Calcitonin Gene-Related Peptide (CGRP) Mechanisms (eds. Brain, S. D. & Geppetti, P.) vol. 255 121–130 (Springer International Publishing, Cham, 2019).
Cann, R. O. et al. Selection of an Enantioselective Process for the Preparation of a CGRP Receptor Inhibitor. Org. Process Res. Dev. 16, 1953–1966 (2012).






